STANDARD OPERATING PROCEDURES FOR CHEMICAL SAFETY
The OSHA Lab Standard identifies eight “elements” that must be included in a Chemical Hygiene Plan. The first of these is Standard Operating Procedures (SOPs) “relevant to safety and health considerations to be followed when laboratory work involves the use of hazardous chemicals.” [29 CFR 1910.1450 (e)(3)(i)] This is especially important if your lab operations include the routine use of particularly hazardous substances, i.e., “select carcinogens,” reproductive toxins, and substances which have a high degree of acute toxicity. [29 CFR1910.1450 (e)(3)(viii)]
The idea of Standard Operating Procedures is certainly not new and is a mandatory item in industry and private research. However, the academic world has been slow to embrace this requirement. Each lab needs to have Standard Operating Procedures that are clear, consist, and useful to lab personnel for training and safety purposes.
SOP’s may be developed according to a process or procedure, to classes of hazardous chemicals, individual hazardous chemicals, or any other reasonable approach that address health and safety concerns of an experimental protocol.
SOP’s need to be tailored for the specific processes of the specific lab. However there are some basic SOP’s that are generally applicable to all labs. A list of these basic standard operating procedures is provided below. EHS is currently preparing draft examples of these SOP, which can be modified to fit the specific laboratory.
As more SOP’s are developed, they will be posted within this site. If you have written one which you would like to share, or if you have questions, or need assistance in the preparation of an SOP, please e-mail Taylor Santaloci
LIST OF SOPS
standard operating procedure
Add Text or Links Here
Add Text or Links Here
Introduction
This standard operating procedure (SOP) is intended to provide general guidance on how to safely work with flammable liquids. This SOP is generic in nature and only addresses safety issues specific to flammable and combustible liquids. In some instances, several general use SOPs may be applicable for a specific chemical.
definitions
National Fire Protection Agency (NFPA)
The definition of a chemical is any chemical with a flashpoint below 37.8ºC (100ºF).
The Classes of Flammable Liquids:
- Class IA. Liquids having a flash point below 73F (23°C) and having a boiling point below 100F (38°C). Examples: acetaldehyde, butyne, chloropropylene, dimethyl sulfide, ethyl chloride, and ethyl ether.
- Class IB. Liquids having a flash point below 73F (23°C) and having a boiling point above 100F (38°C). Examples: acetone, benzene, carbon disulfide, ethyl alcohol, ethyl acetate, gasoline,
- Class IC. Liquids having a flash point at or above 73F (23°C) and below 100F (38°C) Examples: amyl alcohol, butyl alcohol, isobutyl alcohol, methyl isobutyl ketone, styrene, turpentine, and xylene.
OCCUPATION SAFETY AND HEALTH ADMINSTRATION (OSHA)
Definition a flammable liquid as any liquid having a flashpoint at or below 199.4 °F (93 °C).
OSHA FLAMMABLE LIQUIDS FOUR CATEGORIES
- Category 1: shall include liquids having flashpoints below 73.4 °F (23 °C) and having a boiling point at or below 95 °F (35 °C).
- Category 2: shall include liquids having flashpoints below 73.4 °F (23 °C) and having a boiling point above 95 °F (35 °C).
- Category 3: shall include liquids having flashpoints at or above 73.4 °F (23 °C) and at or below 140 °F (60 °C). When a Category 3 liquid with a flashpoint at or above 100 °F (37.8 °C) is heated for use to within 30 °F (16.7 °C) of its flashpoint, it shall be handled in accordance with the requirements for a Category 3 liquid with a flashpoint below 100 °F (37.8 °C).
- Category 4: shall include liquids having flashpoints above 140 °F (60 °C) and at or below 199.4 °F (93 °C). When a Category 4 flammable liquid is heated for use to within 30 °F (16.7 °C) of its flashpoint, it shall be handled in accordance with the requirements for a Category 3 liquid with a flashpoint at or above 100 °F (37.8 °C).
Combustible Liquid: A liquid having a flash point above 100°F. Combustible liquids are subdivided as follows:
List of Combustible Liquids
- Class II: Liquids with a flash point at or above 100°F and below 140°F (60°C).
- Examples:
- No. 1, 2 and 3 fuel oils
- kerosene
- hexyl alcohol
- Examples:
- Class IIIA: Liquids with a flash point at or above 140°F and below 200°F (93°C).
- Examples
- aniline
- benzaldehyde
- butyl cellosolve
- nitrobenzene
- pine oil
- Examples
- Class IIIB: Liquids with a flash point at or above 200°F.
- Examples:
- animal oils
- ethylene glycol
- glycerin
- lubricating quenching, and transformer oils
- triethanolamine
- benzyl alcohol
- hydraulic fluids
- vegetable oils.
- Examples:
Other Definitions
Boiling Point: The temperature at which a liquid’s vapor pressure is equal to the atmospheric pressure. Liquids with low boiling points are very volatile.
Flash Point: The minimum temperature of a liquid at which sufficient vapor is liberated to form a vapor-air mixture that will ignite and propagate a flame away from the ignition source (flash fire not continuous combustion).
Flammable (Explosive) Limits/Flammable (Explosive) Range: The terms flammable and explosive are used interchangeably since unconfined vapors mixed in air will burn while confined vapors will produce an explosion. The minimum vapor concentration in air that, when ignited, will propagate a flame is the lower flammable limit (LFL or LEL). The maximum vapor concentration in air that when ignited will propagate a flame is the upper flammable or explosive limit (UFL or UEL).
Vapor Pressure: A measure of the pressure created by a liquid’s vapor at a specific temperature. Flammable or combustible liquids with a high vapor pressure at room temperature are more hazardous than liquids with lower vapor pressures because they will produce more flammable vapor without heating.
Vapor Density: The weight of a volume of pure vapor or gas (with no air present) compared to the weight of an equal volume of dry air at the same temperature and pressure. A vapor density figure less than one indicates the vapor is lighter than air. A figure greater than one indicates the vapor is heavier than air.
Fire Area: An area of a building separated from the remainder of the building by construction having a fire resistance at least 1 hour (i.e. a single laboratory area).
Flammable Material Storage Cabinet: A storage cabinet constructed and arranged in accordance with NFPA and International Fire Code standards. Note: Cabinets that are typically located underneath bench tops and fume hoods are not considered approved cabinets unless they are provided with appropriate UL/FM labeling.
Flammable Liquid Storage Room: A room used for the storage of large quantities of flammable and combustible liquids which meets the construction, arrangement and protection requirements of the City of Baton Rouge, NFPA and International Building and Fire Code standards.
Safety Can: A metal container of not more than 5-gallon capacity which is UL/FM Approved and is provided with a flame arrestor, a spring-closing lid and spout cover designed to relieve internal pressure when subjected to fire exposure.
Approved Plastic Container: A plastic container meeting the requirements of and containing products authorized by the U. S. Department of Transportation (DOT) Hazardous Materials Regulations, 49 CFR or by Part 6 of the United Nations Recommendations on the Transport of Dangerous Goods (i.e. UN 1H1 – nonremovable head type plastic containers or as authorized by DOT exemption). The 5 gallon “red” container commonly used for gasoline is an example of a container meeting these guidelines.
Potential Hazards/toxicity
- Physical Hazards:
- Flammable liquids usually have high vapor pressures at room temperature and their vapors, mixed with air at the appropriate ratio, can ignite and burn. As with all solvents, their vapor pressure increases with temperature and therefore as temperatures increase, they become more hazardous.
- Combustible liquids require heating for ignition and are easier to extinguish The concentrated vapors of flammable liquids may be heavier than air and can cause vapor trails which can travel to reach an ignition source, resulting in a flashback fire. Fire can also result from reactions between flammables or combustibles and oxidizers.
- Health Hazards:
- In addition to the fire hazard, many flammable and combustible liquids pose health hazards as well. Effects from acute inhalation exposures range from irritation to CNS depression, nausea and dizziness. In extreme situations, coma can result. Chronic exposures may lead to live or kidney damage. Skin absorption can lead to similar longterm effects as inhalation exposures. Skin contact with solvents may result in defatting and drying of the skin. Some flammable liquids also have additional health hazards, i.e., benzene is also a known human carcinogen.
- As the hazards may vary by compound, users must familiarize themselves with the specific hazards of the compounds they are working with, which can be found on the chemical’s Safety Data Sheet (SDS). SDSs are available through the internet using a simple name search.
Personal Protective Equipment (PPE)
The University’s Personal Protective Equipment Policy can be found on the Standard Operating Procedure section of the EHS website.
- Eye Protection:
- Safety glasses must be worn whenever handling flammable liquids. When there is the potential for splashes, goggles and/or a face shield must be worn.
- Hand Protection:
- Gloves must be worn when handling flammable liquids. Exam style nitrile gloves (minimum 4mil thickness) are generally adequate for handling these compounds in laboratory settings when skin contact is unlikely. However, if skin contact is likely or larger amounts are being used, then a utility grade glove should be worn over the exam style nitrile. To ensure that the appropriate utility grade glove is selected, refer to the manufacturer’s glove selection information on the chemical’s SDS, or contact EHS.
- Skin and Body Protection:
- Long pants or clothing that covers the body to the ankles and closed-toe solid top shoes must be worn when handling flammable liquids. Lab coats must be worn. When working with large amounts of flammable liquids, a 100% cotton or flame-resistant lab coat is preferred. For flammable liquids that pose health hazards through dermal absorption, additional protective clothing (i.e., apron, oversleeves) may be appropriate where chemical contact with the skin is likely.
Engineering Controls
- Fume Hood:
- Fume hoods, or other locally exhausted ventilation, should be used whenever handling flammable liquids. Local exhaust ventilation is particularly important when using larger quantities (>500ml) or when flammables are heated or at increased pressure.
- Storage/Handling:
- Minimize the storage of flammable liquids outside flammable rated storage cabinets. The volume stored outside of rated cabinets and safety cans should be <10 gallons per laboratory.
- 5-gallon cans of flammable liquids should be stored inside flammable rated cabinets.
- Refrigerators used for the storage of flammable liquids should be designed/rated for this purpose.
- Keep flammables segregated from incompatible materials, including oxidizers.
- Store at/below eye level (~5 feet).
- Flammable cabinets should be unvented. If venting is required or requested, EH&S must be contacted for a specific evaluation and guidelines.
- Metal surfaces or containers through which flammable liquids flow must be properly grounded, to discharge static electricity.
- Large quantities (≥5 gallons) of flammable liquids should be handled using sparkfree tools in areas free of ignition sources, including spark emitting motors and equipment.
- Never heat flammable liquids by using an open flame. Use steam baths, water baths, oils baths, heating mantles or hot air baths.
- If flammable liquids must be heated in an oven, make sure the oven is appropriately designed for flammable liquids (no internal ignition sources and/or vented mechanically).
- When heating flammable liquids, ensure that the autoignition temperature of the solvent is above the oven temperature or its internal elements.
- Do not distill flammable liquids under reduced pressure.
waste disposal
- Flammable liquids must be collected as hazardous waste including dilute aqueous solutions. Water from the LSU sewers goes to the Baton Rouge sewer system. As such, it is EHS policy that nothing can be disposed by pouring it down the drain. EHS will pick up these solutions for disposal.
- Researchers are not charged for waste collection at LSU.
- In addition, all items contaminated with a flammable liquid which is also acutely toxic (P-Listed) must be collected as hazardous waste, ex: carbon disulfide.
- This includes reagent bottles, weigh boats, pipette tips, kimwipes, and other similar items that have come into contact with these compounds.
Emergency procedure
- Fire Extinguishers:
- Both ABC dry powder and carbon dioxide extinguishers are appropriate for most fires involving acutely toxic compounds. Fire extinguishers should be located within a 50 ft. travel distance.
- Eyewash/Safety Showers:
- An ANSI approved eyewash station that can provide quick drenching or flushing of the eyes should be immediately available within 10 seconds travel time for emergency use. An ANSI approved safety drench shower should also be available within 10 seconds travel time from where these compounds are used. Ensure the locations of the eyewashes and safety showers, and how to activate them, are known prior to an emergency.
first aid procedure
- If Inhaled:
- Remove to fresh air. The employee should notify their supervisor and then call the Employee Injury Call Center at 877-764-3574 to speak to a registered nurse. The Call Center is available 24 hours every day. The nurse will discuss the incident/injury with the employee and determine the employee’s immediate medical needs.
- If Skin Contact:
- Go to the nearest emergency shower if contaminated. Yell for assistance and rinse for 15 minutes, removing all articles of clothing to ensure contaminate is completely removed. The employee should notify their supervisor. Follow up with a call the Employee Injury Call Center at 877-764-3574 to speak to a registered nurse.
- If Eye Contact:
- Go to the nearest emergency eyewash. Yell for assistance and rinse for 15 minutes. . The employee should notify their supervisor and follow up with a call the Employee Injury Call Center at 877-764-3574 to speak to a registered nurse.
spills
- If Small Spill:
- lab personnel should be able to safely clean it up by following standard spill clean-up
procedures:
- Alert people in immediate area of spill
- Increase ventilation in area of spill (open fume hood sashes)
- Wear personal protective equipment, including utility grade gloves
- Confine/adsorb spill with spill clean-up pads or absorbent
- Collect residue, place in container, label container, and dispose of as hazardous waste
- Clean spill area with soap and water Larger Spill/Any spill outside a fume hood
- If necessary, call EHS for emergency assistance (225-578-5640).
- lab personnel should be able to safely clean it up by following standard spill clean-up
procedures:
- If Large Spill/Any Spill outside of fume hood
- Call LSU Police for emergency assistance at 225-578-3231
- Notify EHS (225-578-5640).
- Evacuate the spill area
- Post someone or mark-off the hazardous area with tape and warning signs to keep other people from entering
- Stay nearby until emergency personnel arrive and provide them with information on the chemicals involved
PURPOSE
Proper storage is needed to minimize the hazards associated with accidentally mixing incompatible chemicals. Due to the diverse individual properties of chemicals that may be located in a chemical use area, proper storage may be complicated. This SOP provides general safety procedures for chemical storage. Specific instructions on chemical storage may be obtained from the MSDS, container label, or by contacting OES.
SCOPE
This procedure applies to all Louisiana State University Personnel that use and handle chemicals. It is the intent of this guideline to provide information on the safe storage of chemicals and afford employee protection from potential health and physical hazards associated with accidentally mixing incompatible chemicals.
RESPONSIBILITIES
Only trained and qualified personnel shall be allowed to handle hazardous materials. Supervisors are responsible for ensuring that personnel are trained to handle chemicals and that all chemical are store in a safe manner. The chemical incompatibilities discussed below are by no means exhaustive. As a result, it is important for laboratory personnel to thoroughly research the properties of the chemicals they are using. Material Safety Data Sheets (MSDSs) have sections on chemical incompatibility. The container's label should also provide storage guidelines
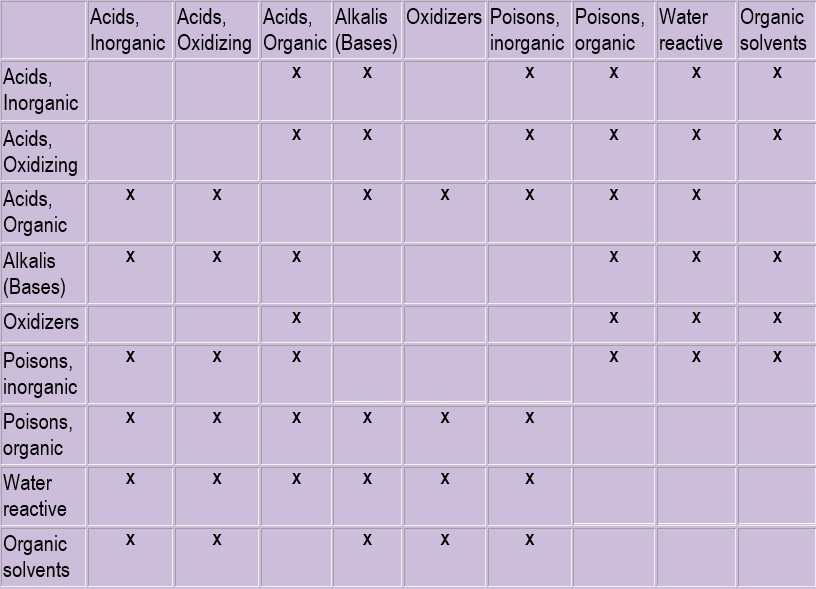
DEFINITIONS
- Pyrophoric Substances: Materials which will react with the air to ignite when exposed, e.g., white phosphorus.
- Oxidizing Agent: Reactive material that oxidizes another substance and is reduced.
- Acid: Corrosive material that that produces H+ (aq) ions in aqueous solution. Strong acids ionize completely or almost completely in dilute aqueous solution. Weak acids ionize only slightly.
- Base: Corrosive material that produces OH (aq) ions in aqueous solution. Strong bases are soluble in water and are completely dissociated. Weak bases ionize only slightly.
- Flammable: A liquid as defined by NFPD and DOT as having a flash point below 37.8°C (100°F).
References
- Manufacturing Chemists' Association, Guide for Safety in the Chemical Laboratory, pp. 215217, Van Nostrand Reinhold, 2nd Edition
- "Safe Chemical Storage: A Pound of Prevention is Worth a Ton of Trouble" by David Pipitone and Donald Hedberg, Journal of Chemical Education, Volume 59, Number 5, May 1982 "Fire Protection Guide on Hazardous Materials," NFPA, 1978
materials and/or equipment
- Proper personal protective equipment (PPE)
- Gloves
- Long Pants
- Closed Toe Shoes
- Safety Glasses or Goggles
- Shirts with Sleeves
procedures
- Know the properties of the used chemicals.
- In general, chemicals should be separated according to the following categories:
- Solvents, which include flammable/combustible liquids and halogenated hydrocarbons (e.g., acetone, benzene, ethers, alcohols) Note: Store glacial acetic acid as a flammable liquid
- Inorganic mineral acids (e.g., nitric, sulfuric, hydrochloric, and perchloric acids).
- Bases (e.g., sodium hydroxide, ammonium hydroxide)
- Oxidizers
- Poisons
- Explosives or unstable reactives, such as picric acid. Store separately outdoors in flammable storage cabinets. An inventory of all chemicals must be maintained. Inventories must include the full chemical name, location of storage, and associated hazard (e.g. corrosive or flammable
- Inventories must be updated annually and signed by the person performing the update. Chemicals purchased throughout the year must be added to the inventory as soon as they are brought into the work area. Post chemical inventories inside the room with a hazard summary posting on the door for emergency response purposes.
- Ensure that all containers are in good condition, properly capped, and labeled. If you are using short hand names or acronyms on any solutions, reagents, or aliquots of chemicals, you must maintain a cross-reference sheet that defines the short hand name or acronym in use such as EtOH = ethanol or PBS=phosphate buffered saline. Review this list annually to ensure that all short hand names or acronyms in use are recorded.
- Store all hazardous liquid chemicals in drip trays that are chemically resistant. Photo trays provide good containment and are widely used at the Lab. Install Plexiglas lips or use equivalent means to prevent materials from falling off storage shelves.
- Avoid storing chemicals on countertops or in fume hoods except for those being currently used.
- Avoid storing chemicals above eye level. Select low shelves or cabinets for heavy containers. Never store chemicals or any other item closer than 18” to the ceiling. Storing an item close to the ceiling will impede the effectiveness of automatic fire suppression systems.
- Do not store chemicals on the floor. Chemical containers could present a tripping hazard or could be knocked over causing a spill.
- Label all containers (squeeze bottles and Nalgene bottles) to which hazardous materials are transferred with the identity of the substance and its hazards. Be aware that squeeze bottles and Nalgene bottles have varying resistances to different chemicals.
- Limit the amount of chemicals stored to the minimum required.
- Avoid exposure of chemicals to heat or direct sunlight. This may lead to the deterioration of storage containers as well as the degradation of the chemicals.
- Use approved corrosive storage cabinets (constructed of chemically resistant components) for storing acids and bases.
- Use flammable storage cabinets to store flammable liquids.
- Refrigerators used for storing chemicals, samples or media must be labeled with words to the effect as follows: “Caution – Do Not Store Food Or Beverages In This Refrigerator”. Refrigerators used for food storage in or near work areas (shops and labs) must be labeled with words to the effect as: “Notice – Food May Be Stored In this Refrigerator”. Labels may be fabricated by users provided they are legible and securely affixed to the refrigerator. Refrigerators used for food and beverage which are located in lunch rooms and office buildings, where there is no shop or lab type chemical usage do not require any posting.
- Refrigerators and freezers for storing flammable liquids (including ethanol) must be designed, constructed and approved for that purpose. Domestic refrigerator/freezers as well as units that have been modified to remove spark sources are not acceptable alternatives.
- Avoid mixing incompatible waste materials. A number of serious laboratory accidents have occurred when people have poured incompatible waste materials into hazardous waste containers. Use separate waste containers for each type of waste.
storage according to hazard classes
The following guidelines are provided for the safe storage of hazardous materials in accordance with their hazard classes:
- Acids
- Segregate acids from reactive metals such as sodium, potassium, magnesium.
- Segregate oxidizing acids from organic acids, flammable and combustible materials.
- Segregate acids from chemicals which could generate toxic or flammable gases upon contact, such as sodium cyanide, iron sulfide, calcium carbide
- Segregate acids from bases.
- Bases
- Segregate bases from acids, metals, explosives, organic peroxides and easily ignitable materials.
- Solvents (Flammable and Halogenated Solvents)
- Store in approved safety cans or cabinets.
- Segregate from oxidizing acids and oxidizers.
- Keep away from reducing agents such as zinc, alkali metals, and formic acid.
- Oxidizers
- Store in a cool, dry place.
- Keep away from combustible and flammable materials.
- Keep away from reducing agents such as zinc, alkali metals, and formic acid.
- Cyanides
- Segregate from acids and oxidizers.
- Water Reactive Chemicals
- Store in a cool, dry place away from any water source.
- Make certain that a Class D fire extinguisher is available in case of fire.
- Pyrophoric
- Store in a cool, dry place making provisions for an airtight seal.
- Light Sensitive Chemicals
- Store in amber bottles in a cool, dry, dark place.
- Peroxide Forming Chemicals
- Store in airtight containers in a dark, cool, and dry place
- Label containers with receiving, opening, and disposal dates.
- Periodically test for the presence of peroxides.
- Toxic Chemicals
- Store according to the nature of the chemical, using appropriate security where necessary.
contingencies
- In case of a Fire, Explosion, or Gas leak:
- Evacuate individuals from the area and call the emergency response (911). Notify supervision and adjacent personnel as quickly as possible. Observe appropriate procedures for personal injury or fire as provided in EHS Web site.
- In case of a Chemical Spill:
- Determine the severity of the spill and proceed as appreciate. Small spills may be cleaned up by laboratory personnel.
- For a small spill, may be cleaned up by laboratory personnel.
- For a Large spill, notify EHS (578-5640) and Campus Police (911 or 578-3231). If possible to do so safely (without risk of overexposure), take action to stop the release. Ensure that extraneous personnel remain at a safe distance until the spill is completely cleaned-up
reviews and revisions
This procedure shall be reviewed for compliance and effectiveness and revised as necessary on an annual basis.
attachments and reference forms
EVALUATION OF POTENTIAL AND KNOWN HAZARDS
Prior to initiating a new experiment or procedure, all laboratory employees must evaluate the potential physical and health hazards associated with its chemicals and processes. Container labels and material safety data sheets, as well as other references, will be used to conduct the evaluation. Laboratory personnel will be familiar with their own and previous evaluations prior to beginning work and will use appropriate ventilation, protective equipment, and procedures to minimize exposure. The evaluation will include preparation for any potential emergency.
SUBSTITUTION AS A PRIMARY METHOD OF CONTROL
Following hazard evaluation, laboratory personnel should always consider substituting less hazardous and toxic substances. Only chemicals for which appropriate exposure controls are present may be used.
PRIOR ARROVAL
Laboratory employees must obtain prior approval to proceed with a laboratory task from a supervisor whenever:
- a new laboratory procedure or test is carried out.
- There is a significant change in a procedure or test likely to alter the hazard. A significant change is defined as a 10% or greater increase or decrease in the amount of one or more chemicals used; a substitution or deletion of any of the chemicals in a procedure; or a change in the conditions under which the procedure is conducted.
- There are unknown or unexpected test results.
- Members of the laboratory staff become ill, suspect exposure, or otherwise suspect failure of the engineering safeguards.
Reporting Laboratory Incidents and Unsafe Conditions
Report all laboratory incidents no matter how minor to a supervisor. Incident report forms are available from the Chemical Safety Manager or Environmental Health and Safety Manager in the Department of Environmental Health and Safety. Unusual or unexplainable chemical incidents should be discussed with others in the department, to caution others as to the risk of the procedure.
Report any unsafe conditions by contacting the Chemical Safety Manager or the Environmental Health and Safety Manager. File a written report so that the conditions may be corrected as soon as possible.
Unsafe conditions which must be reported include:
- Non-functioning hoods in areas where hazardous chemicals are being used.
- Fume hoods that are out of yearly certification date.
- Unsafe storage conditions.
- Blocked emergency exits
- Improperly charged fire extinguishers
- Inoperable eyewash stations or safety showers
- Absence of Personal Potective Equipment (PPE) (e.g goggles, gloves).
general rules
Working with hazardous chemicals (and procedures) alone in a laboratory or chemical storage area is strictly PROHIBITED. Lab personnel must schedule research and experiments involving hazardous substances and procedures so that other lab members are present.
Rules are as follows:
- Undergraduate teaching laboratories: A faculty member must be present in the lab at all times when undergraduate students are conducting experiments.
- Research Laboratories: Personnel working alone must contact Public Safety to make them aware of their presence in the facility and encourage them to periodically check on them. This personnel should plan a route of escape in case of an emergency.
- Always wear appropriate eye protection.
- When working with flammable chemicals, be certain that there are no sources of ignition near enough to cause a fire or explosion in the event of a vapor release or liquid spill.
- Use a tip-resistant shield for protection whenever an explosion or implosion might occur.
For the chemicals they are using, all employees should be aware of:
- The chemicals' hazards, as determined from the MSDS and other appropriate references.
- Appropriate safeguards (e.g. chemical fume hood, personal protective equipment, etc.).
- The location(s) and proper use of emergency equipment (e.g. emergency shower/eyewash, fire extinguisher, spill kit).
- How and where to properly store the chemical when it is not in use.
- Proper personal hygiene practices.
- The proper methods of transporting chemicals within the facility.
- Appropriate procedures for emergencies, including evacuation routes, spill cleanup procedures, and proper waste disposal.
Personal Hygiene
- Never store food or beverages in storage areas, refrigerators, glassware, or use utensils that are also used for laboratory operations
- Do not eat, drink, smoke, chew gum, or apply cosmetics in laboratories where chemicals or other hazardous materials (e.g., radioactive or biohazards) are present.
- Never mouth pipet. Always use a pipet bulb or other mechanical pipet-filling device.
- Do not smell or taste chemicals.
- Wash areas of exposed skin well before leaving the laboratory.
- Confine long hair and loose clothing. Wear close-toed shoes at all times in the laboratory. Sandals and perforated shoes are prohibited. Tennis shoes are not appropriate for work with hazardous chemicals.
- Always wear clothing that completely covers chest, arms, and legs. While performing laboratory work, never wear short-sleeved T-shirts, V-neck shirts, or other low-cut shirts exposing the chest, crop tops, short skirts, or shorts.
- Jewelry that interferes with gloves and other protective clothing or that could meet electrical sources or react with chemicals must be removed.
Proper Equipment Use
- Only use the equipment for its intended purpose.
- Inspect equipment or lab apparatus for damage before use. Never use damaged equipment such as cracked glassware, or equipment with frayed electrical wiring.
- Shield or wrap Dewar flasks and other evacuated glassware to contain chemicals and glass fragments should implosion occur.
Personal protective equipment (ppe)
Choose protective clothing and other equipment based on the types of chemicals handled, the degree of protection required, and the areas of the body which may become contaminated. All clothing and equipment must at a minimum, meet standards set by the American National Standards Institute (ANSI). All respiratory protective equipment must be chosen in conjunction with the Industrial Hygiene Manager since there are strict legal requirements as to the use and distribution of these devices.
Every effort must be made to evaluate the effectiveness of equipment and make improvements where possible. Special consideration must be given to purchasing appropriate PPE and other safety equipment when extremely hazardous substances are involved. The choice of this equipment under these circumstances must be reviewed by the Chemical Safety Manager in advance of purchase requests.
Eye Protection
All personnel, students, and any visitors in locations where chemicals are stored or handled must always wear protective goggles. Setting the requirements for their use is the responsibility of lab supervisors and directors. All eyewear must meet the American National Standards Institute's (ANSI) Practice for Occupational and Educational Eye and Face Protection, Z87.11989. Prior to use, personnel will verify that the equipment has been approved for the procedure (e.g., eye protection may be ANSI certified for chemical splashes but not for explosions). ANSI standards require a minimum lens thickness of 3mm, impact resistance, the passage of a flammability test, and lens-retaining frames.
Contact lenses can be used in laboratories. Recent studies By the Centers for Disease Control and Prevention have produced varying views on the issue of contact lens use in laboratories. Traditional safety lore claimed that contaminated aerosols or particulate matter would concentrate behind contact lenses and cause permanent eye damage. In contradiction to this assumption, some researchers have found that contact lenses may minimize injuries to the eye from metal particles, paint fumes, and chemical splashes from solvents and acids. Working in a properly performing chemical fume hood must always be the first line of defense against chemical exposure. The following table will be consulted in choosing protective eyewear.
| Type of Eye Protection | Condition |
|---|---|
| Acid/Caustic goggles with side shields | Danger of splashing chemicals or flying particles |
| Impact protection goggles | Working with glassware under vacuum or elevated pressures; using glass apparatus in combustion or other high-temperature operations |
| Face shields (protect the face, throat and neck) | Potential for flying particles, harmful liquid |
| Specialized eye protection | lasers; intense ultraviolet and other light sources; glass blowing |
ORDINARY PRESCRIPTION GLASSES ARE NOT ADEQUATE TO PROTECT EYES FROM INJURY!
Guidelines for Use of Gloves
Gloves must be worn whenever there is a chance for hand contact with chemicals, such as during the transfer of chemicals from one container to another or during the transfer of chemical waste. Gloves must be worn if the chemicals involved are easily absorbed through the skin and/or are acute or chronic toxins. When working with corrosive liquids, always wear gloves made of a material known to be resistant to permeation by the corrosive chemical and tested by air inflation for the absence of pin-hole leaks.
Lab personnel must inspect gloves prior to each use. Non-disposable gloves must be washed before removal except those that are easily permeated/degraded by water (e.g. leather, polyvinyl alcohol).
Prior to use, lab personnel will consult the glove manufacturer’s permeation and resistance charts (available from the manufacturer) to make sure that the glove is appropriate for the chemicals being used. Glove materials vary in the way they resist being degraded and permeated. No glove totally resists degradation and permeation over time and must be replaced periodically, depending on the frequency of use, chemical concentration, and duration of contact. The glove material and its thickness determine the appropriateness of a specific glove type.
Clothing
The choice of protective clothing depends upon the degree of protection required. Protective and appropriate clothing is required when a potential exists for chemical splashes, fire, extreme heat or cold, excessive moisture, and radiation. Setting requirements for their use is the responsibility of lab supervisors and directors.
Protective clothing which should be readily available to laboratory personnel include:
- Lab Coats
- Boots
- Lab Aprons
- Shoe Covers
- Gauntlets
- Jumpsuits/coveralls
Laboratory personnel must be instructed to consider the following characteristics in protective clothing selection and purchase:
- Ability to resist fire, heat, and the chemicals used. (For example, if a flash fire hazard may be present, clothes that are made of natural fiber are much better than those of synthetic fiber. In the presence of fire, synthetic fibers may melt and cause more sever burns).
- Impermeability, when needed.
- Comfort, permitting easy execution of tasks when worn.
- Ease of cleaning (unless disposable).
- Ability to be removed quickly during an emergency or chemical splash (e.g. snap fasteners rather than buttons).
Safety Shields
Safety shields should be used on or near the equipment when there is potential for explosion, implosion, or splash hazards. Fixed shields will be used whenever possible, recognizing that their weight and resistance provide superior protection against minor blasts. Portable shields may be used when the hazard is limited to small splashes, heat, or fire. Where combustion is possible, the shield must be made of flame-resistant material. It is the laboratory supervisor's responsibility to ensure that shields are used appropriately. The sash of a chemical fume hood can serve as a splash or (minor) blast shield.
Prior to large volume purchases, personal protective equipment should be evaluated under real or simulated conditions to ensure that it meets both safety and performance standards. For example, chemical splash goggles may meet ANSI standards but fog up rapidly or are so uncomfortable that they will not be worn.
Respirators
OSHA requires all employers to primarily prevent air contamination. If vapor concentrations cannot be kept below regulated levels, the employer must implement a written respirator program (29 CFR 1910.134). The written program will address issues such as respirator selection criteria, inspection, and maintenance. All personnel using respirators must be trained in their proper use and care. Additionally, medical evaluation and proper fit testing are required. However, respirator use is considered the very last line of defense against chemical exposures. Engineering controls, changes in work practices, chemical substitution, and changes in experimental procedures must be employed before respirator use will even be considered. EH&S will determine if respirator use is required and will determine the proper respiratory protection equipment to be used if it is determined that it is indeed necessary.
Transporting chemicals within LSU (between rooms and/or between buildings)
- Carry chemicals by hand in secondary containment (carrying bucket) to prevent breakage or spillage.
- Transport chemicals on stable, wheeled carts that move smoothly over uneven surfaces; cart shelving should have raised edges to contain chemicals if containers break.
- Laboratory employees transporting chemicals must wear goggles.
- Use freight elevators whenever possible, passenger elevators only during periods of low use.
- Transport compressed gas cylinders using hand trucks with the cylinder strapped in place. NEVER roll or drag cylinders. Keep the cylinder capped until used.
Housekeeping
- Keep all work areas, including workbenches and floors, clean, dry, and uncluttered.
- Access to emergency equipment, utility controls, showers, eyewash stations, and laboratory exits must never be blocked.
- Label all chemical containers with the full chemical name(s) of the contents and hazards.
- Return all chemicals to their assigned storage areas at the end of each workday.
- Properly label all waste containers.
- Promptly clean up all chemical spills; properly dispose of the spilled chemical, and cleanup materials.
- Chemicals must be stored in proper secondary containment, in cabinets with closeable doors, or chemical shelving (storage rooms).
Working with Toxic Chemicals
Laboratory personnel must be aware of the physical properties (reactivity, corrosivity, flammability) of the chemicals they use. They are often not aware of the toxicology of these same chemicals. MSDSs (and SDSs) will state several exposure limits (if established) for a specific chemical, such as Threshold Limit Values (TLV, American Conference of Governmental Industrial Hygienists, ACGIH), Permissible Exposure Limits (PEL, OSHA), and Action Levels. When such limits are stated, they will be used to assist the Chemical Safety Manager in determining the safety precautions, control measures, and PPE that apply when working with toxic chemicals. Chemicals must be used in a properly-operating fume hood, glove box, vacuum line, or similar device, which is equipped with appropriate traps and/or scrubbers if:
- The TLV or PEL < 50 ppm or 50 mg/m3.
- The Lethal Concentration (LC50) < 200ppm or 200mg/m3 (when administered continuously for one hour or less).
- The chemical is highly volatile and likely to exceed maximum air concentration limits.
- EHS decides controls are required.
Deposit chemical waste in their appropriate, labeled, receptacles and follow all other disposal procedures described in LSU’s Chemical Hygiene Plan. Be particularly cautious about releasing hazardous substances into designated cold rooms or warm rooms since these facilities have recirculated atmosphere.
Minimize the release of toxic vapors into the laboratory by venting apparatus such as vacuum pumps and distillation columns into the local exhaust system (i.e. chemical fume hoods). When toxic or corrosive vapors are involved, they should pass through scrubbers prior to being discharged from the local exhaust system.
Working with Flammable Chemicals
- In general, the flammability of a chemical is determined by its flash point, the lowest temperature at which an ignition source can cause the chemical to ignite momentarily under certain controlled conditions.
- Chemicals with a flash point below 200OF (93.3OC) will be considered "fire-hazard chemicals" (flammable or combustible).
- In all work with fire-hazard chemicals, follow the requirements of
- 29 CFR, Subparts H, and L
- NFPA Manual 30
- Flammable and Combustible Liquids Code
- NFPA Manual 45, Fire Protection for Laboratories Using Chemicals.
- Fire-hazard chemicals must be stored in a flammable storage cabinet.
- Fire-hazard chemicals must be used only in chemical hoods, away from sources of ignition.
Working with Reactive Chemicals
- The definition of a reactive chemical:
- is described as such in the SDS.
- is ranked by the NFPA as 3 or 4 for reactivity.
- is identified by the Department of Transportation (DOT) as an oxidizer, an organic peroxide, or an explosive, Class A, B, or C.
- meets the EPA definition of reactive in 40 CFR 261.23.
- meets the OSHA definition of unstable in 29 CFR 1910.1450.
- is known or found to be reactive with other substances.
- Handle reactive chemicals with all proper safety precautions:
- Segration in storage
- Prohibition on mixing even small quantities with other chemicals without prior approval
- Appropriate PPE
- Precautions
- Work practices
Working with Corrosive and Contact Hazard Chemicals
- Corrosivity, allergenic, and sensitizer information is sometimes provided on manufacturers' SDSs and labels.
- Guidelines on corrosive chemicals can be found in
- OSHA standards
- DOT in 49 CFR
- EPA in 40 CFR
PURPOSE
Use of an appropriate ensemble of personal protective equipment (PPE) creates a second line of defense against exposure to hazardous chemicals. Engineering controls, such as fume hoods and other ventilation devices, are used to create a first line of defense. When engineering controls are not adequate to minimize exposures to acceptable levels, LSU departments must provide employees with adequate PPE. Protective equipment shall be used and maintained in sanitary and reliable condition. Under no circumstances shall a person knowingly be subjected to a hazardous condition without appropriate personal protective equipment. Components selected for an adequate ensemble of PPE vary with the route and degree of exposure. General classes of PPE, with specific examples, are discussed below. Upon request, EHS will provide guidance on the selection of the appropriate classes and specific types of PPE. This SOP provides general safety procedures for the use of personal protective equipment.
Scope
This procedure applies to all Louisiana State University Personnel that work in a
laboratory. It is the intent of this guideline to provide information on the general
PPE of a laboratory at LSU and afford employee protection while working in a laboratory.
Responsibilities
Only trained and qualified personnel shall be allowed to work in a laboratory at LSU. Persons who are exposed to hazards requiring personal protective equipment shall be properly instructed in the use of such equipment by the individual in charge of the activity or his/her designee. It is the responsibility of the individual in charge of the activity to assure that safety practices are adhered to. If those individuals required to wear personal protective equipment fail to do so, they will be subject to disciplinary action.
Definitions
Not Applicable
References
Not Applicable
Materials and/or Equipment
Not Applicable
Procedure For Personal Protective Equipment
Procedure for Respirator Usage
The use of respirators in a laboratory setting is not acceptable as a standard practice.
Engineering controls are required to reduce air contaminants to an acceptable level.
Voluntary respirator use in a laboratory setting may be acceptable under special conditions.
Many types of respirators are available, and the appropriate type of respirator depends
on the concentration of contaminants, as well as the form of contaminants (e.g., dusts,
mists, fumes, etc.). If considering respirator use, contact EHS because there are
medical, training, and fit test requirements for respirator use. Refer to the LSU
Safety Manual for additional information.
Eye and Face Protection
Safety glasses with side shields, goggles, or face shields are required when there
is potential for exposures to chemical splashes or fumes, dusts, flying projectiles,
heat, or optical radiation. All protective eyewear must meet the American National
Standard for Eye Protection for Occupational and Educational Eye and Face Protection
Z87.1
Approved eyewear is required while working in a laboratory.
Management level employees, students, or visitors who make occasional visits to labs
shall wear approved eyewear.
Prescription lens wearers, if required to wear eye protection, such shall wear an
approved face shield, goggles that fit over glasses, prescription glasses with protective
optical lenses fitted with side shields, or goggles that incorporate prescription
lenses.
Contact lenses shall never be considered as a substitute for eye protection; eye protection
shall be worn over them.
All eye and face protection shall be kept clean and inspected daily before use. Badly
scratched or damaged items are to be replaced immediately.
Hand Protection
Gloves provide protection for the hands from many types of hazards, including chemical
absorption. Like other classes of PPE, many types of gloves are available, ranging
in material of construction and thickness. Selection of an appropriate glove depends
on specific chemicals to which the user is or may be exposed, as well as severity
of exposure (e.g., incidental, or low hazard contact verses immersion of the hands,
or high hazard contact) and manual dexterity considerations.
Hand protection shall be worn by employees when handling hot work, chemicals, electrical,
material handling of rough and/or sharp items, doing landscaping work, welding, and
"wherever it is necessary by reason of hazards of processes of environmental, chemical
hazards, radiological hazards, or mechanical irritants encountered in a manner capable
of causing injury or impairment." (OSHA 1910 Standards)
Hand protection used will meet the criteria for its particular use. Consult with
EHS for assistance in selection as required. The EHS web site contains chemical resistance
charts for glove selection. Other factors such as durability, dexterity, frequency
and ease of donning and removing gloves are also important factors in glove selection.
Gloves shall be selected to fit comfortably and snugly.
All hand protection shall be kept clean and inspected daily before each use. Badly
worn or damaged items are to be replaced.
All glove manufactures provide permeability data for specific gloves. Manufacturers
may show different data for the same glove material. It is imperative to review
this data before selecting the appropriate glove. For Glove selection, consult the
Occupational and Environmental Safety Web Page or contact EHS.In general, examination-type
gloves are very thin and provide protection only for incidental contact (e.g., unexpected
small droplets). These types of gloves are disposable and should be removed immediately
upon contamination, with the hands washed immediately after removal. It is best to
avoid gloves constructed of latex because of associated allergy hazards.
Silvershield gloves provide the broadest range of possible protection but are not
suitable for operations where the hands are immersed in a chemical or when dexterity
is of great importance.
Long, thick gloves, constructed of butyl rubber or other material depending on the
chemical of interest, must be used when immersing the hands in chemical solutions.
Always try to avoid immersion of the hands in any chemical solution, regardless of
glove use, by implementing engineering solutions (e.g., retrieval tongs, removable
baskets, etc.).
Protective and Preventive Clothing
Protective body apparel is required when there is potential for accidental spills
or splashes. Material of construction varies with the type of garment selected. Cotton,
flame-retardant laboratory smocks or coats provide protection in low hazard situations.
More sophisticated apparel, such as Tyvek coveralls, may be necessary when working
with large quantities or highly dangerous chemicals.
Protective clothing shall be worn by employees/students when the potential of an employee/student being exposed or coming in contact with harmful substance is evident. i.e., chemicals, high heat (radiant), dust, open flame, etc.
There are many different standards for approval of protective clothing (ANSI, ASTM,
etc.). Protective clothing shall be selected for specified hazard, degree of protection,
comfort, and ease of use. Once the specific or multi hazards have been identified,
contact a reputable vendor Environmental Health and Safety personnel for recommendation
of proper protective clothing and/or equipment needed.
Protective clothing shall fit the wearer comfortably and shall not be too loose or
baggy.
Protective clothing shall be routinely cleaned unless disposable. Disposable clothing
shall be disposed of after use. Damaged, torn, ripped, etc., clothing shall be replaced
before use.
Foot Protection
Protective footwear should be selected based on the degree of hazard.
Street shoes are generally sufficient to provide protection in low-hazard operations
(e.g., laboratory scale). Bare feet, sandals, and open-toed shoes are not permitted
when working with chemicals.
Shoe covers provide protection in medium-hazard operations (e.g., contact with chemicals
is likely but risk of splash is low). Selection of the material of construction for
shoe covers is very important. Like gloves, the material of construction and thickness
determines the level of protection of the shoe cover.
Formed boots provide the highest level of protection and are designed for operations
with significant potential for contact with chemicals. Formed boots may also be necessary
for medium-hazard activities that are not compatible with shoe covers because of the
likelihood of damage to the shoe cover (e.g., outdoors, abrasive floor coverings,
etc.) and for activities that require good footing (e.g., slippery surfaces). Consult
the manufacturer’s permeability data when selecting the material of construction and
follow the manufacturer’s recommendations for cleaning or discarding.
All foot protection shall be kept reasonably clean and in good repair. Shoes shall
be repaired or replaced periodically.
Hearing Protection
Protective hearing should be selected based on the potential noise level.
Employees who are exposed to hazardous levels of noise in the workplace are at risk
for developing noise‐induced hearing loss. Noise‐induced hearing loss is fully preventable
but once acquired, hearing loss is irreversible. While the noise levels in most laboratories
are below the threshold level that damages hearing, laboratory noise can be fairly
loud. Various laboratory equipment contribute to the noise level. If you feel that
your lab has a noise problem, contact EHS to conduct a noise survey. If noise levels
exceed 85 dBA, hearing protection is required.
Headphones or earbuds use for entertainment is not allowed in the laboratory. You
must be able to hear what is going on around you. These devices make it very hard
to hear emergency alerts such as fire alarms. Another concern is that headphone/earbud
use makes it difficult for workers to remain “aware of their surroundings” and “maintain
an appropriate level of safety consciousness.” Other forms of music/radio may be
played in the lab but nothing requiring the use of headphones.
Contingencies
In case of a fire, explosion, or gas leak evacuate individuals from the area and call
the emergency response (911). Notify supervision and adjacent personnel as quickly
as possible. Observe appropriate procedures for personal injury or fire as provided
on the EHS Web site.
In case of a chemical spill, alert others in the immediate vicinity and notify your supervisor. Determine the severity of the spill and proceed as appreciate. Small spills may be cleaned up by laboratory personnel. For large spills, notify EHS (578-5640) and Campus Police (911 or 578-3231). If possible, to do so safely (without risk of over-exposure), take action to stop the release. Ensure that extraneous personnel remain at a safe distance until the spill is completely cleaned-up.
Reviews And Revisions
This procedure shall be reviewed for compliance and effectiveness and revised as necessary
on an annual basis.
Attachments and Reference Forms
Not Applicable
PURPOSE
PURPOSE
PURPOSE
PURPOSE
PURPOSE
PURPOSE
PURPOSE
PURPOSE
purpose
click here for pdf version This SOP describes methods for safely using, storing, and disposing of Hydrofluoric acid. Hydrofluoric acid, known as HF, is an extremely corrosive acid used for many purposes including mineral digestion, surface cleaning, etching and biological staining. HF’s unique properties make it significantly more hazardous than many of the other acids used in laboratories.
HF is very aggressive physiologically because of the fluoride ion. Both anhydrous hydrofluoric acid and its solutions are clear, colorless liquids. When exposed to air, concentrated solutions and anhydrous hydrofluoric acid produce pungent fumes which are especially dangerous.
WARNING: Burns with concentrated HF are usually very serious, with the potential for significant complications due to fluoride toxicity. Concentrated HF, liquid or vapor, may cause severe burns, metabolic imbalances, pulmonary edema and life threatening cardiac arrhythmias. Even moderate exposures to concentrated HF may rapidly progress to fatality if left untreated.
scope
This procedure applies to all Louisiana State University Personnel that use and handle hydrofluoric acid. Every effort must be made to prevent exposure to HF. Following this guideline will provide the information and tools to protect you and assist in getting necessary medical treatment in the event of an exposure.
responsibilities
- Principle Investigators/Supervisors:
- Shall ensure that this guideline is read and implemented in their work areas and labs. All work areas using HF will be marked to alert all persons of the presence of HF. They shall ensure that all workers using HF receive the appropriate training before using HF and maintain documentation of the training. PIs will provide all workers with the MSDS, protective equipment and warning signs for HF. PIs will ensure the HF is added to the on-line chemical inventory system upon receipt.
- Hydrofluoric Acid (HF) Users:
- Shall ensure this guideline is read and implemented in all work areas and labs that are using, storing, and disposing of HF. All users will attend a documented training session on the proper handling, storage and first aid procedures for HF. All users will read the MSDS for HF, know the first aid/medical treatment procedures and spill response for HF.
- The Environmental Health & Safety (EHS) Office:
- Will act as a reference source for HF information and maintain the chemical inventory system.
health hazard data
- Inhalation:
- Severely corrosive to the respiratory tract and may cause sore throat, coughing, labored breathing and lung congestion/inflammation.
- Skin Contact:
- Corrosive to the skin. Skin contact causes serious skin burns which may not be immediately apparent or painful. Symptoms may be delayed 8 hours or longer. The fluoride ion readily penetrates the skin causing destruction of deep tissue layers and even bone. Eye Contact: Corrosive to the eyes. Symptoms of redness, pain, blurred vision, and permanent eye damage may occur.
- Eye Contact:
- Corrosive to the eyes. Symptoms of redness, pain, blurred vision, and permanent eye damage may occur.
- Chronic Exposure:
- Intake of more than 6 mg of fluorine per day may result in fluorosis, bone and joint damage. Hypocalcaemia and hypomagnesaemia can occur from absorption of fluoride ion into blood stream.
- Aggravation of Pre-existing Conditions:
- Persons with pre-existing skin disorders, eye problems, or impaired kidney or respiratory function may be more susceptible to the effects of this substance.
first aid
- Skin Exposure Procedure:
- Move victim immediately under safety shower or other water source and flush affected area. Speed and thoroughness in washing off the acid is of primary importance. Individuals assisting victim should wear appropriate gloves to prevent secondary HF burn.
- Remove clothing while continuing to flush with water.
- Rinse with large amounts of running water for 2-5 minutes. Apply a 2.5% calcium gluconate gel to the affected area. Massage gel into the burn site; apply frequently and continuously until pain and/or redness disappear or until medical help arrives.
- While victim is being treated, someone should call LSU police (911 on a land line or 225-578-3241 on a cell phone) and request immediate medical assistance/transport to Emergency Room (ER). Hospital ER must be notified that an HF victim will be arriving for immediate treatment.
- Victim must be transported to hospital for additional examination and/or treatment.
- Provide EMT/transport personnel with HF MSDS or other chemical information for hospital.
- Eye Contact Procedure:
- Immediately flush the eyes for at least 15 minutes with large amounts of gently flowing water. Hold the eyelids open and away from the eye during irrigation to allow for thorough flushing of the eyes.
- If the person is wearing contact lenses, the lenses should be removed, if possible. However, flushing with water should not be interrupted. Do not use skin treatment gel for eye contact burns.
- While victim is being treated, someone should call LSU police (911 on a land line or 225-578-3241 on a cell phone) and request immediate medical assistance/transport to Emergency Room (ER). Hospital ER must be notified that an HF victim will be arriving for immediate treatment.
- Victim must be transported to hospital for additional examination and/or treatment.
- Provide EMT/transport personnel with HF MSDS or other chemical information for hospital.
- Inhalation of Vapors Procedure
- Immediately move victim to fresh air and get medical attention.
- Keep victim warm, quiet and comfortable. If breathing has stopped, start CPR.
- Check victim for vapor burns to the skin and treat as in Skin Exposure Procedure
- While victim is being treated, someone should call LSU police (911 on a land line or 225-578-3241 on a cell phone) and request immediate medical assistance/transport to Emergency Room (ER). Hospital ER must be notified that an HF victim will be arriving for immediate treatment.
- Victim must be transported to hospital for additional examination and/or treatment.
- Provide EMT/transport personnel with HF MSDS or other chemical information for hospital.
- Ingestion of Acid
- Have the victim drinks large amounts of water as quickly as possible to dilute acid. DO NOT induce vomiting. Never give anything by mouth to an unconscious person.
- Give several glasses of milk or several ounces of milk of magnesia or grind up and administer 30 antacid tablets with water. The calcium or magnesium in these compounds may act an antidote.
- While victim is being treated, someone should call LSU police (911 on a land line or 225-578-3241 on a cell phone) and request immediate medical assistance/transport to Emergency Room (ER). Hospital ER must be notified that an HF victim will be arriving for immediate treatment
- Victim must be transported to hospital for additional examination and/or treatment. Ingestion of HF can be life threatening.
- Provide EMT/transport personnel with HF MSDS or other chemical information for hospital.
safety controls
All personnel who work with HF should undergo safety training prior to working with this chemical, have access to and have read the Material Safety Data Sheet (MSDS), review and understand this guideline and understand emergency first aid treatment for HF exposure.
- Substitution:
- Determine if a less hazardous substance can be substituted for HF or use a less concentrated solution in the applied research or application.
- Work in Fume Hood:
- All HF work must be conducted in a designated and properly functioning fume hood.
- Safety Equipment:
- The area must be equipped with a safety shower (may be in hall) or drench hose (within lab space) and HF spill kit, which includes a 2.5% calcium gluconate gel. (The PI must ensure that the calcium gluconate gel is not expired.)
- Warnings:
- Post warning signs on Space Hazard Placard for presence of HF and on designated fume hood.
- General Safety:
- Do not work alone, if possible.
- Do not eat, drink or smoke where HF is handled.
- Wash hands thoroughly after handling (after glove removal).
- Store according to proper procedures (see section title STORAGE REQUIREMENTS).
- Transport in an appropriate secondary container.
- Ensure a working phone is in the lab space.
personal protective equipment (ppe)
Be sure that you are using personal protective equipment that has been shown to effectively protect against HF exposure. Always double check your equipment before each use of HF.
- Respiratory Protection:
- HF should be used within a properly functioning fume hood to minimize inhalation of vapor. The hood sash should be kept as low as possible.
- Eye Protection
- All users must wear a full-face shield when working in a hood with the sash pulled down below the face. This will prevent splash hazards in a spill situation. Splash goggles must be worn with a full-face shield when work is performed outside of a hood. Safety glasses with side protectors do not protect from splashes.
- Body Protection:
- Wear a laboratory coat with a chemical splash apron made out of neoprene or Viton®. Never wear shorts, skirts, or open toed shoes in the lab or when handling HF.
- Hand Protection:
- Chemical Protective Gloves must be worn for laboratory use of HF. Nitrile gloves (22 ml) are sufficient for use but should be double gloved. Other gloves recommended for use with HF that do not require double gloves include Neoprene or Viton®
- If possible, double glove at all times to protect against pin holes or tears.
- DO NOT use latex gloves; they are not effective against HF.
- Discard used gloves, after rinsing with water, into a trash receptacle. If gloves are significantly contaminated with HF, discard into a hazardous waste disposal container to prevent secondary contamination to persons using regular trash receptacles.
- Thoroughly wash hands after glove removal and check hands for any sign of contamination.
storage requirements
- HF should be stored in its original container with all markings intact.
- Hydrofluoric acid should be kept in tightly closed polyethylene containers. These containers should be stored in a cool, dry, well-ventilated place away from other chemicals. Secondary containment should be used. Empty containers can be hazardous, since residual vapors or liquid may be present.
- HF transfer from larger container to a smaller container should take place in a designated fume hood. The smaller container must be identified with the chemical hazard label according to labeling procedures in LSU Chemical Hygiene Plan. The smaller container must be of a compatible material to hold and store the HF.
Note: Do not use squirt or squeeze bottles because overpressure may force HF out of the nozzle. It is recommended to use a dropper bottle in these situations.
spill cleanup
- Definitions:
- Small Spill:
- a small incidental chemical spill that the user can clean up safely, easily and without assistance from emergency response personnel.
- Large/Hazardous Spill:
- a chemical spill requiring the assistance of the emergency response team or outside responders.
- Small Spill:
- Small Spill:
A small incidental spill of HF, in the work area, may be cleaned by the user if they are knowledgeable of the material and have the ability and equipment to safely accomplish this In the event of a spill, consult the MSDS again to ensure that you and others follow the recommended cleanup procedures. HF spill kits are required in areas where the chemical is used.
SMALL SPILL CLEANUP PROCEDURE
- Attend to any person that has been exposed to HF, utilizing emergency drench hoses, eyewashes, or showers, and phoning for assistance. Follow first aid procedures in Section 5.
- Alert all other personnel that may be affected by the spill.
- Keep fume hoods operating to remove vapors.
- Keep fume hoods operating to remove vapors.
- Limit the area of the spill as much as possible by using absorbent materials that are available in the HF spill kit (see section 9.4 for minimum contents).
- After cleanup, manage the contaminated materials as hazardous waste and request a waste pickup (OES@lsu.edu or x5640). See section 10 for procedures.
- Hazardous/Large Spill:
A hazardous spill is any spill of HF that is not considered by the user to be an incidental spill.
HAZARDOUS/LARGE SPILL CLEANUP PROCEDURE
- Report the emergency by calling 911 on a land line (cellular phones call 225-578-3231
- Warn others in the spill area to evacuate. Generally, the spill area is defined by
the extent and location of the spill. For example:
- if the spill is within the lab - evacuate the lab
- if the spill is within the hall – evacuate immediate area of hall.
- Evacuate to a safe area and barricade the area to prevent entry to spill area.
- Attend to any person that has been exposed to HF if safe to do so (See section title FIRST AID).
HF SPILL KIT CONTENTS
- 2.5% calcium gluconate gel
- Antacid tablets (TUMS – Calcium Carbonate Tablets)
- Hydrofluoric acid MSDS
- Commercial Color-change neutralizer with instructions
- Nitride gloves
- Apron
- Shoe Covers
- Hazardous waste container and tag
- Spill scoop and brush
waste disposal
HF is a corrosive substance and must be collected for disposal. Use a polyethylene container for collected HF waste and ensure cap is closed tightly. Place in a plastic bag to store. Complete the chemical waste tag, affix to container, and request a waste pickup on the EHS website.
PURPOSE
PURPOSE