Q&A with LSU Neuroscientist Dr. Nicolás Bazan on Alzheimer’s Disease, Risk, and Hope
November 10, 2025

Dr. Nicolás Bazan, Boyd Professor of Neuroscience at LSU Health New Orleans and founding director of the LSU Neuroscience Center
November is National Alzheimer’s Disease Awareness Month, and the timing is fitting. The days are getting shorter, impacting our circadian rhythms and sleep. Holidays are approaching, which often bring a mix of joy and stress, relaxation and loneliness. The end of the year will also remind us of our New Year’s health goals.
Myth
Alzheimer’s is a natural part of aging.
Reality
We can meaningfully decrease our risk and/or delay the development of Alzheimer’s disease through our actions. Early detection and risk reduction are crucial.
Risk Factors
What do all of these aspects of life—sleep, social interaction, stress, depression, goals, and healthy habits—have in common? These are all factors that can impact our risk of developing dementia.
While many of us focus on our risk factors for dementia, such as our family history, we may not focus as much on our resilience factors. There are steps we can take to protect our brains as we age, starting as early as our 30s and 40s.
In this Q&A, Dr. Nicolás Bazan, Boyd Professor of Neuroscience at LSU Health New Orleans and founding director of the LSU Neuroscience Center, reflects on his journey into brain research, what’s changing in the Alzheimer’s disease research field, what we know about risk and resilience factors, and where new therapies may come from.
Bazan recently published a new book, Brain Aging and Resiliency, that explores how the brain adapts to stress and how we can harness brain factors of resilience to ward off disease. We asked him about his research.
Q&A
How did you become interested in brain research and, ultimately, Alzheimer’s disease?
My interest started with a deeply personal experience. As an 8-year-old in Argentina, my aunt was walking me to a piano lesson when she collapsed from a grand mal seizure. My mother later explained that my aunt had a disorder called epilepsy. That single event planted a lifelong question: How does the brain maintain its integrity when confronted with challenges?
My fascination with the brain led me to medical school and drove me to search for the molecular guardians that protect the brain. Many of these guardians are lipids, which are fats that represent the brain’s most abundant molecules after water.
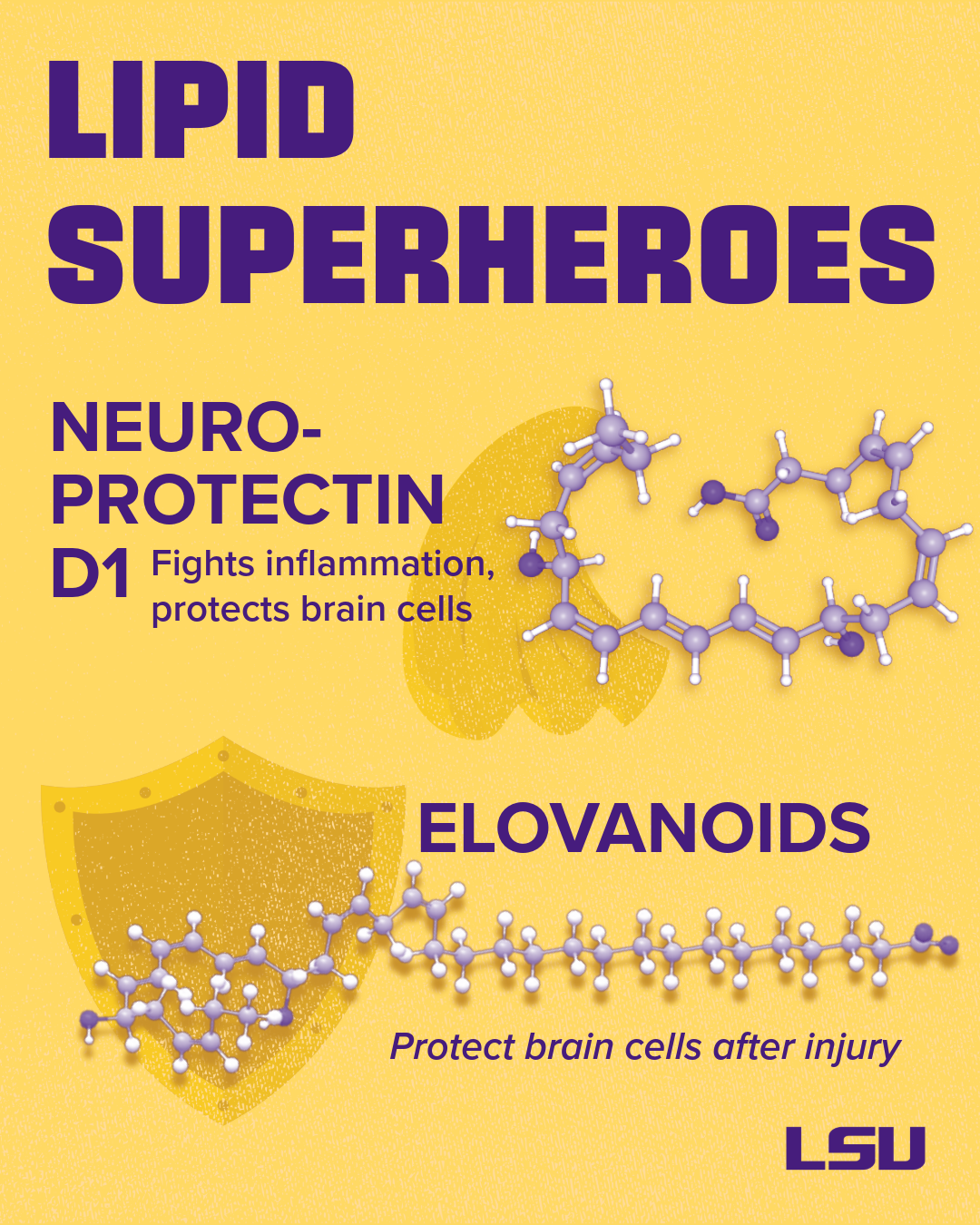
My lab has explored lipid-derived “stay alive” signals for neurons. Among these, we discovered Neuroprotectin D1 and the elovanoids, which are derived from omega-3 fatty acids. These lipids help maintain and sustain the survival and connections between your brain cells, called neurons.
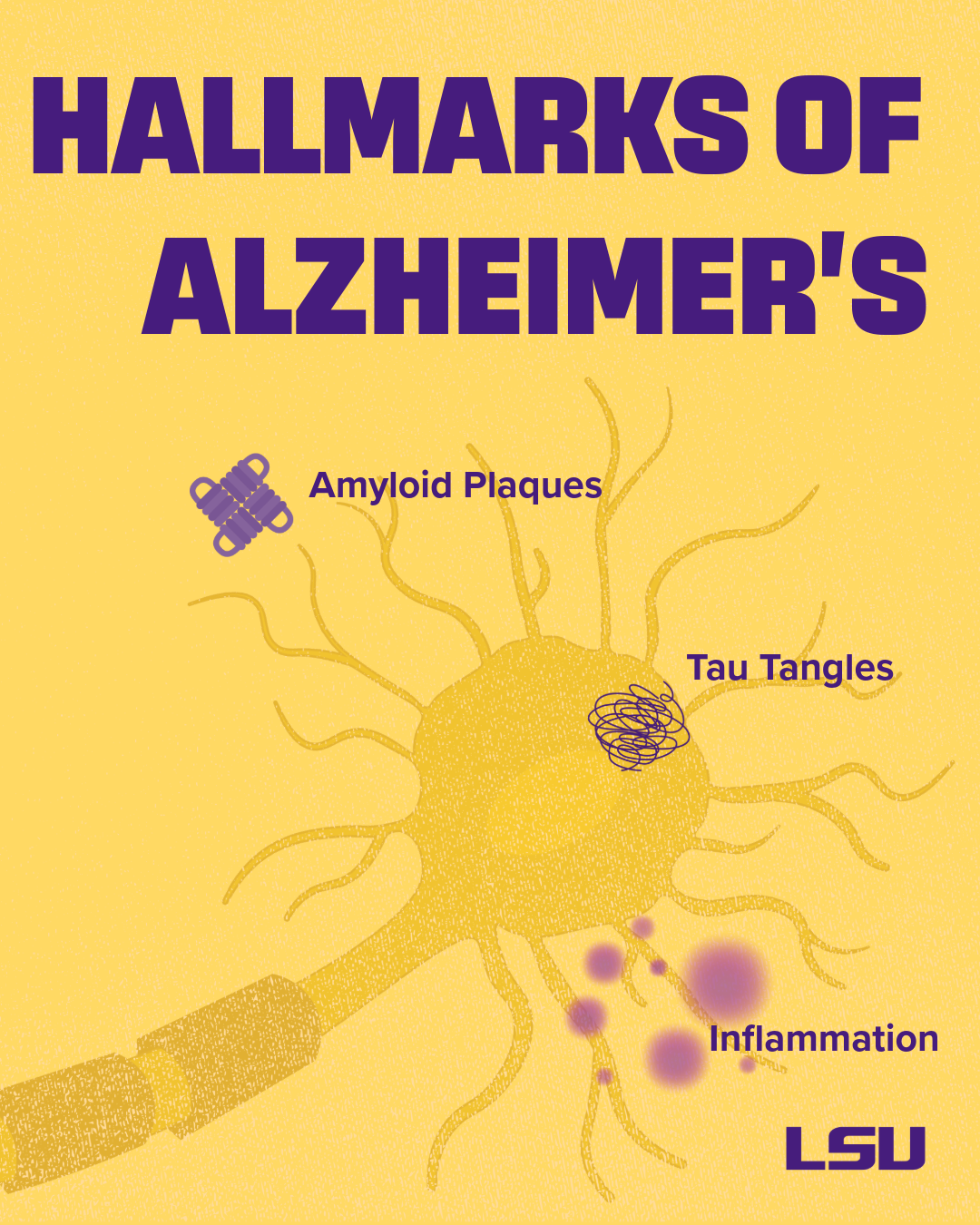
Today, we investigate how these lipids are formed at the onset of injury, how they influence gene expression, and how they interact with Alzheimer’s disease-related triggers, such as amyloid plaques, tau tangles, oxidative stress, and neuroinflammation. Our central question is: how can we harness and amplify the brain’s own protective mechanisms to delay or prevent neurodegeneration?
Editor’s note: Bazan and collaborators found that neuroprotectin D1, derived from the omega-3 essential fatty acid docosahexaenoic acid (DHA), is anti-inflammatory and inhibits the creation of sticky amyloid-beta peptides, key components of Alzheimer's disease plaques. Learn more about the research.
What are the brain’s “molecular guardians,” and can we boost them?
The “guardians” or neuroprotection mediators we’ve studied, such as neuroprotectin D1 and elavanoids, are formed from precursors stored in cell membranes, very-long-chain polyunsaturated omega-3 fatty acids. These mediators are only produced and released in bursts when needed; however, larger amounts are released early after a stroke and/or other brain injury.
Neuroprotectin D1 and elovanoids are potent; they contribute to resolving inflammation, restoring protein balance, and activating survival genes that stabilize connections between brain cells. Yet, they are typically only temporarily available. We can enhance their production by ensuring an adequate precursor supply (e.g., through diets rich in omega-3 fatty acids) and by developing therapeutic analogs that mimic their effects. Identifying these pathways opens the door to treatments that enhance the brain’s resilience, complementing existing therapies that aim to clear the brain protein aggregates.
One of the precursors for neuroprotectin D1 is DHA, a structural fatty acid found in brain cell membranes, especially at the ends of brain cells (synapses) and in the eye’s light receptors. Neuroprotectin D1 dampens inflammatory genes, inhibits the creation of “sticky” amyloid proteins that clump together, and promotes cell survival.
Dietary DHA from fatty fish or supplements provides precursors for the creation of neuroprotectin D1; however, the mode and timing of delivery matter. In my lab, we’re advancing targeted forms and synthetic analogs of beneficial lipids, for example, a form of elovanoids that can be delivered to the brain via nasal spray.
But overall, what we are learning is that a balanced diet is the right approach, including vegetables, fruits rich in antioxidants, plus wild-type salmon.
Editor’s note: In neurodegenerative disease, pathological cascades overwhelm the brain’s molecular guardians. However, a healthy lifestyle and future therapeutic interventions might boost the numbers of these beneficial lipids.
How has our scientific understanding of Alzheimer’s disease changed over the years?
We’ve moved from viewing Alzheimer’s as a single-pathway disease to seeing a problem created by a complex interplay of many biological systems. Proteins like tau become misfolded, the brain’s immune system is dysregulated, brain blood vessels are altered, and metabolism changes.
All of these changes contribute to the development of the disease. But one of the rising hallmarks of Alzheimer’s disease and related dementias is unresolved, chronic inflammation.
Myth
Amyloid plaques alone cause Alzheimer’s disease.
Reality
Many molecular and metabolic changes are associated with Alzheimer’s disease.
How are protein aggregation and inflammation connected to dementia?
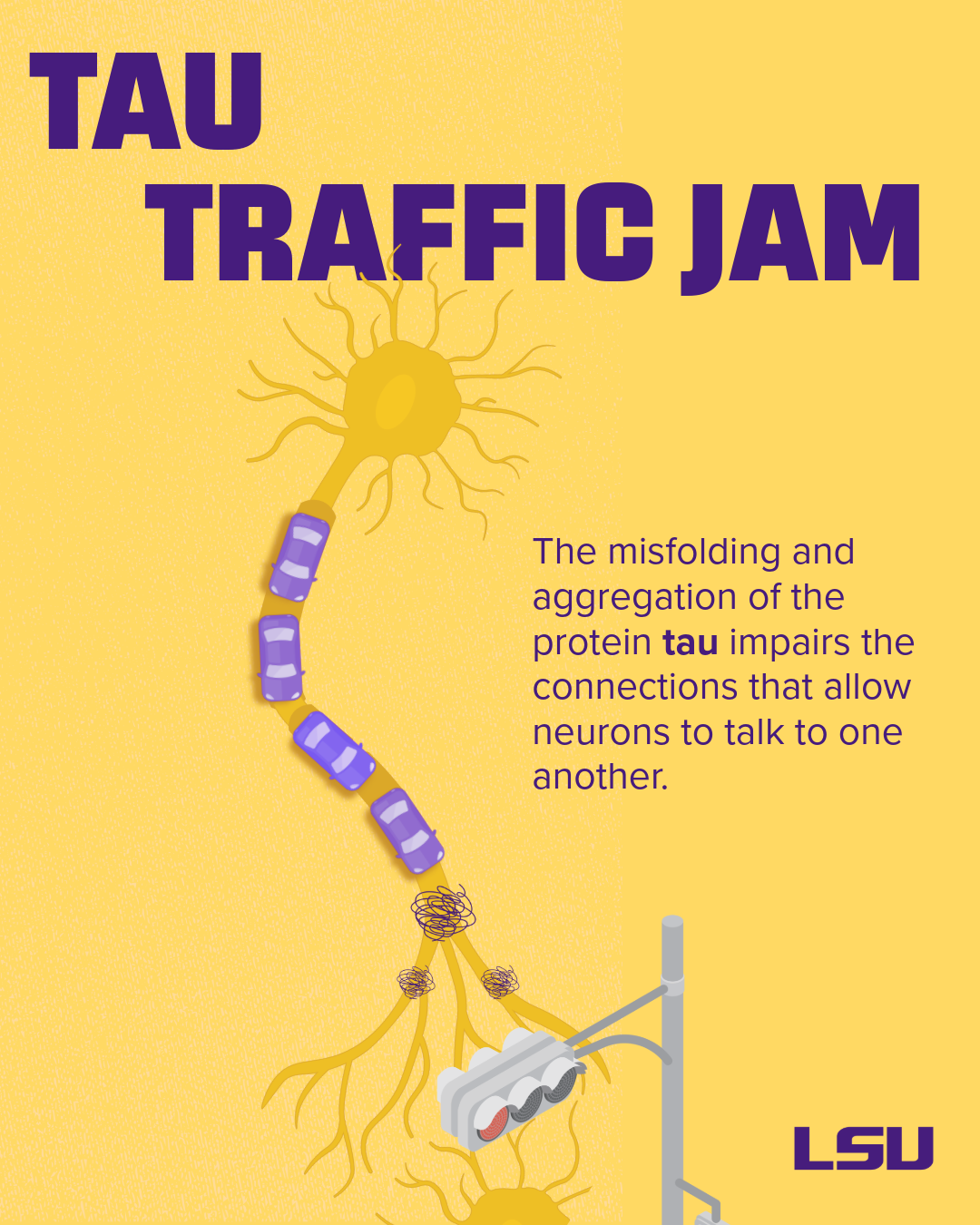
Proteins that are normal in healthy brains can misfold, clump together, and become toxic. Protein aggregates of amyloid-beta, tau, or alpha-synuclein disrupt critical cell functions.
Problems with tau protein folding and aggregation are known as tauopathies – Alzheimer’s disease is a type of tauopathy. Misfolded or “pathological” tau can spread between neighboring neurons, leading to a decline in neurological function.
Misfolded tau and other misfolded proteins can also trigger the activation of damaging forms of microglia, the brain’s immune cells, leading to inflammation. If the body fails to resolve this inflammation, it becomes chronic, leading to synaptic loss (the loss of communication connections between neurons) and impaired neuronal networks.
Diabetes, obesity, periodontal disease, and chronic infections can exacerbate brain inflammation. These pathologies prime microglia to be inflammatory and compromise the blood-brain barrier, increasing the brain’s vulnerability. However, our research shows that omega-3-derived mediators can help restore balance and promote brain cell survival, highlighting the importance of systemic health in brain resilience.
Myth
Alzheimer’s disease really starts when symptoms appear.
Reality
Biological changes associated with disease can begin 20–30 years before memory problems appear. These changes are detectable in cerebrospinal fluid, blood, and brain images. Because no cure yet exists, early detection and risk reduction efforts are key.
What risk factors are within our control?
Age remains the strongest risk factor, but genetics, including the APOE4 gene, influences
susceptibility. However, genes are not destiny. Environmental and lifestyle factors
such as cardiovascular health, sleep quality, education, cognitive engagement, and
exposure to air pollution also play critical roles.
Think of risk as a dynamic balance: Genetics may load the dice, but lifestyle choices
determine how they roll. While absolute prevention may not be possible, modifying
your risk factors by exercising, eating a healthy diet rich in fruits and vegetables,
engaging socially, managing blood pressure and cholesterol, and avoiding smoking can
substantially reduce your likelihood of developing Alzheimer’s disease.
What do you feel are some of the most promising directions in the field right now?

There are many promising directions, including prevention based on an individual’s
unique risk factors, earlier detection, combination therapies that address multiple
disease mechanisms, interventions targeting risk genes like APOE4, and therapeutics
that boost resilience pathways and lipids.
There are also many challenges: We need to start treatment early enough to be effective.
We need to understand how the disease presents differently in different people. We
need to find ways to deliver drugs across the blood-brain barrier. We need to better
understand the impact of sleep on the brain. We also need to ensure equitable access
to emerging therapies.
To overcome these hurdles, we must align biomarkers, timing, and multi-target strategies in adaptive and inclusive clinical trials. The research in our lab includes understanding how our organs function as a highly coordinated system. One example is the ‘brain–heart axis,’ which shows how neuroscience and cardiology work together.
In the “broken heart syndrome” (Takotsubo cardiomyopathy), acute emotional stress can cause cardiac dysfunction. If we can decode other brain–organ axes and the body’s complex networks, we can develop interventions that extend not only lifespan but also health-span.
In a paper appearing soon in the Nature Journal Scientific Reports, my lab found that changing the gut microbiota (the balance of all the microbes living in the gut) and lipid metabolism in mice with genetic risk for Alzheimers’s disease, by feeding them a diet high in fiber, offers a promising, non-invasive strategy to prevent or slow disease progression.
This work represents a transformative step forward in understanding the brain–gut axis and could help reduce the onset and progression of Alzheimer’s disease, and likely Parkinson's as well.
Myth
We have a cure for Alzheimer’s disease.
Reality
Most current therapies work by targeting amyloid protein plaques in the brain. They show promise but limited clinical benefits. Some believe this is because the drugs are typically given once the disease is advanced, and patients often have unaddressed co-pathologies that are fueling inflammation and brain cell injury. Long clinical trial durations, safety concerns, and inadequate outcome measures have made the development of new drugs difficult.
What can we do as individuals to build our resiliency for successful brain aging?
Everyone can build resilience and enjoy the journey of aging. Successful aging is
rooted in resilience, purpose, and proactive health measures. In my book, I explore
how attitude, dedication, and lifestyle choices, as well as arts such as music, influence
cognitive health.
Individuals with a strong sense of purpose are more likely to adopt healthier habits
and seek early interventions, thereby delaying the onset of disease.
- Prevention and proactive measures should also start early in life. Here are some actions:
- Get lots of cognitive stimulation in midlife
- Use hearing protection and hearing aids as needed
- Manage depression
- Wear a helmet to prevent head injuries
- Avoid smoking
- Control blood pressure and cholesterol from midlife
- Maintain a healthy weight
- Reduce your alcohol intake
- Foster supportive, age-friendly communities to reduce isolation
These steps will enhance brain resilience and promote successful aging.
Bazan has founded three startup biopharmaceutical companies—NeuResto Therapeutics LLC, CurVir Biotech LLC, and South Rampart Pharma Inc.—each of which licenses patents from his laboratory. With over 100 patents describing novel molecules and mechanisms, these companies aim to bring innovative therapies to clinical trials. South Rampart Pharma, for instance, has received FDA approval for Phase II development, marking a step toward real-world impact.
Next Step
Discover stories showcasing LSU’s academic excellence, innovation, culture, and impact across Louisiana.


